-
Entrada Therapeutics Presents New Data Supporting its Growing Pipeline of Endosomal Escape Vehicle (EEV™) Therapeutics at TIDES USA 2022
来源: Nasdaq GlobeNewswire / 11 5月 2022 09:30:00 America/New_York
New non-human primate data demonstrate a durability of response through 12 weeks for lead clinical candidate, ENTR-601-44, for the potential treatment of Duchenne muscular dystrophy
Second clinical candidate, ENTR-701, announced for the potential treatment of myotonic dystrophy type 1
On track to submit Investigational New Drug applications to the U.S. Food and Drug Administration for ENTR-601-44 targeting Duchenne muscular dystrophy in Q4 2022 and for ENTR-701 targeting myotonic dystrophy type 1 in 2023
BOSTON, May 11, 2022 (GLOBE NEWSWIRE) -- Entrada Therapeutics, Inc. (Nasdaq: TRDA), a biopharmaceutical company aiming to transform the lives of patients by establishing intracellular Endosomal Escape Vehicle (EEV™) therapeutics as a new class of medicines, today presented updates to its two lead programs at TIDES USA 2022: Oligonucleotide & Peptide Therapeutics Conference. The company announced new non-human primate (NHP) data demonstrating durability of response through 12 weeks for ENTR-601-44, an EEV-conjugated phosphorodiamidate morpholino oligomer (PMO) for the potential treatment of people with Duchenne muscular dystrophy (DMD) who are exon 44 skipping amenable. Entrada also announced its second clinical candidate, ENTR-701, an EEV-PMO that the company is developing as a potential allele-specific treatment for people living with myotonic dystrophy type 1 (DM1).
“We are proud to present new data at TIDES USA for our lead clinical candidate, ENTR-601-44 for Duchenne muscular dystrophy, and to announce our clinical candidate for myotonic dystrophy type 1 as we continue to expand our pipeline of EEV therapeutics,” said Natarajan Sethuraman, PhD, Chief Scientific Officer of Entrada. “To date, we have generated robust in vitro and in vivo preclinical data supporting the advancement of our DMD and DM1 programs. These encouraging data reinforce the potential of our EEV therapeutic candidates to engage previously inaccessible and undruggable disease-causing targets within cells, and we look forward to presenting additional data at upcoming scientific meetings.”
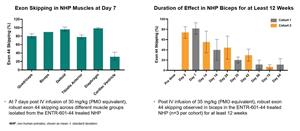
The new data from a preclinical study evaluating ENTR-601-44 for the potential treatment of DMD, show robust exon 44 skipping in NHP biceps through 12 weeks following a single intravenous (IV) infusion, demonstrating durability of response (See Figure 1). These data build on a previously reported NHP study indicating robust exon 44 skipping across different muscle groups at 7 days following a single IV infusion.
Figure 1: A single IV dose of ENTR-601-44 resulted in robust exon skipping in both skeletal and cardiac muscles in NHP, as well as prolonged duration of effect for at least 12 weeks
The selection of ENTR-701 as Entrada’s clinical candidate for DM1 was supported by new preclinical data indicating prolonged splicing correction in the tibialis anterior, triceps and quadriceps, and amelioration of myotonia in a DM1 mouse model following a single dose.
The presentation, entitled “Endosomal Escape Vehicle (EEV)-Conjugation Enhances Functional Delivery of Oligonucleotides,” given by Leo Ziqing Qian, PhD, Co-Founder and Vice President, Discovery Research at Entrada, will be made available on the Publications & Conferences section of the Entrada website following the conference.
About Duchenne Muscular Dystrophy (DMD)
Duchenne muscular dystrophy is a rare, genetic disease that causes progressive muscle degeneration and weakness throughout the body. DMD is caused by mutations in the DMD gene, which leads to inadequate production of dystrophin, a protein essential to maintaining the structural integrity and function of muscle cells. DMD causes progressive loss of muscle function throughout the body, which limits mobility and causes heart and respiratory complications in the later stages of the disease. Currently approved therapies for DMD seek to improve dystrophin production, but to date, the clinical benefits of these products have not been confirmed.About ENTR-601-44
ENTR-601-44, a proprietary Endosomal Escape Vehicle (EEV™)-conjugated phosphorodiamidate morpholino oligomer, is the first novel clinical candidate from Entrada’s growing pipeline of EEV therapeutics. ENTR-601-44 is designed to address the underlying cause of Duchenne muscular dystrophy by skipping the mutated or missing exons in pre-mRNA inherent to DMD. ENTR-601-44 has the potential to restore the mRNA reading frame and allow for the translation of dystrophin protein that is slightly shortened but still functional. Entrada expects to file an IND application with the U.S. FDA for ENTR-601-44 for the potential treatment of patients with Duchenne muscular dystrophy who are amenable to exon 44 skipping in Q4 2022.About Myotonic Dystrophy Type 1 (DM1)
Myotonic dystrophy type 1 is a multi-systemic neuromuscular disease that causes progressive muscle loss and weakness, leading to physical impairment, activity limitations and decreased participation in social activities and work. DM1 is an autosomal dominant disease caused by a mutation to the dystrophia myotonica protein kinase gene, which leads to toxic mRNA that sequesters and reduces function of the muscle blind-like proteins. This causes mis-regulation of multiple RNA splicing events that are correlated with DM1 symptoms, which include muscle weakness, myotonia, cardiac conduction abnormalities, respiratory muscle impairment, gastrointestinal complications and fatigue. DM1 can occur at any age, however, classic disease onset typically occurs in the 20s and 30s and life expectancy can range from 45 to 60 years, with 70% of early mortality caused by cardiac or respiratory complications. There are currently no approved therapies for people living with DM1. Instead, treatment is focused largely on symptom management.About ENTR-701
ENTR-701, a proprietary Endosomal Escape Vehicle (EEV™)-conjugated phosphorodiamidate morpholino oligomer, is the second novel clinical candidate from Entrada’s growing pipeline of EEV therapeutics. ENTR-701 is designed to address the underlying cause of myotonic dystrophy type 1 through allele-specific targeting and blocking of the excess repeat-containing transcripts in dystrophia myotonica protein kinase mRNA. In doing so, ENTR-701 has the potential to restore the function of muscle blind-like proteins, correct the mis-splicing and aberrant expression of downstream transcripts and restore normal muscle function. Data from preclinical studies of ENTR-701 suggest correction of disease relevant biomarkers in various muscle groups. Entrada expects to file an IND application with the U.S. FDA for ENTR-701 in 2023.About Entrada Therapeutics
Entrada Therapeutics is a biopharmaceutical company aiming to transform the lives of patients by establishing a new class of medicines, Endosomal Escape Vehicle (EEV™) therapeutics, to engage intracellular targets that have long been considered inaccessible and undruggable. The Company’s EEV therapeutics are designed to enable the efficient intracellular delivery of a wide range of therapeutics into a variety of organs and tissues with an improved therapeutic index. Through its proprietary, highly versatile and modular EEV platform, Entrada is building a robust development portfolio of oligonucleotide-, antibody- and enzyme-based programs for the potential treatment of neuromuscular diseases, immunology, oncology and diseases of the central nervous system. The Company’s lead oligonucleotide programs include ENTR-601-44 targeting Duchenne muscular dystrophy (DMD) and ENTR-701 targeting myotonic dystrophy type 1 (DM1).For more information about Entrada, please visit our website, www.entradatx.com, and follow us on Twitter and LinkedIn.
Forward-Looking Statements
This press release contains forward-looking statements that involve substantial risks and uncertainties. All statements, other than statements of historical facts, contained in this press release, including statements regarding Entrada’s strategy, future operations, prospects and plans, objectives of management, the continued development of ENTR-601-44, including the Investigational New Drug (IND) application-enabling studies, the timing of Entrada’s planned regulatory filings regarding its development programs, expectations regarding the preclinical data of ENTR-601-44 and ENTR-701 and the related potential for development, the progression of early-stage oligonucleotide, antibody and enzyme-based programs into clinical development, the continued development and advancement of ENTR-601-44 for the treatment of DMD and ENTR-701 for the treatment of DM1, the potential therapeutic benefits of its EEV candidates, and the sufficiency of its cash resources, constitute forward-looking statements within the meaning of The Private Securities Litigation Reform Act of 1995. The words “anticipate,” “believe,” “continue,” “could,” “estimate,” “expect,” “intend,” “may,” “might,” “objective,” “ongoing,” “plan,” “predict,” “project,” “potential,” “should,” or “would,” or the negative of these terms, or other comparable terminology are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words. Entrada may not actually achieve the plans, intentions or expectations disclosed in these forward-looking statements, and you should not place undue reliance on these forward-looking statements. Actual results or events could differ materially from the plans, intentions and expectations disclosed in these forward-looking statements as a result of various important factors, including: uncertainties inherent in the identification and development of product candidates, including the conduct of research activities and the initiation and completion of preclinical studies and clinical trials; uncertainties as to the availability and timing of results from preclinical studies; the timing of and Entrada’s ability to submit and obtain regulatory clearance for IND applications and initiate clinical trials; whether results from preclinical studies will be predictive of the results of later preclinical studies and clinical trials; whether Entrada’s cash resources will be sufficient to fund the Company’s foreseeable and unforeseeable operating expenses and capital expenditure requirements; uncertainties associated with the impact of the ongoing COVID-19 pandemic on Entrada’s business and operations; as well as the risks and uncertainties identified in Entrada’s filings with the Securities and Exchange Commission (SEC), including the Company’s most recent Form 10-K and in subsequent filings Entrada may make with the SEC. In addition, the forward-looking statements included in this press release represent Entrada’s views as of the date of this press release. Entrada anticipates that subsequent events and developments will cause its views to change. However, while Entrada may elect to update these forward-looking statements at some point in the future, it specifically disclaims any obligation to do so. These forward-looking statements should not be relied upon as representing Entrada’s views as of any date subsequent to the date of this press release.Entrada Investor/Media Contact
Karla MacDonald
VP, Corporate Communications and Investor Relations
kmacdonald@entradatx.comA photo accompanying this announcement is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/3bdb84d6-2f78-440f-91b6-a8d42b93f1d6